Introduction:
Chimeric antigen receptor (CAR) T cell therapy has emerged as an important treatment option for patients with B-cell malignancies. Despite its major clinical impact, CAR T cell therapy holds a potential risk of increased susceptibility to infections, including invasive fungal infections (IFI). Since data about the prevalence and impact of fungal infections in this population are limited, we aimed to provide a comprehensive overview of IFI using real-world data from the national DESCAR-T registry.
Methods:
We performed a multi-center retrospective cohort study, quering the French DESCAR-T registry (NCT04328298), to describe type, frequency and outcomes of IFI in adults with refractory or relapsed (R/R) B cell lymphomas. In addition, healthcare providers were contacted to declare any missing cases. Demographic, biological and clinical characteristics were collected retrospectively. Each IFI case was reviewed by an infectious disease specialist and a microbiologist, and classified according to the European Organization for Research and Treatment of Cancer (EORTC) criteria (Donnelly et al. Clinical Infect Dis 2020).
Results:
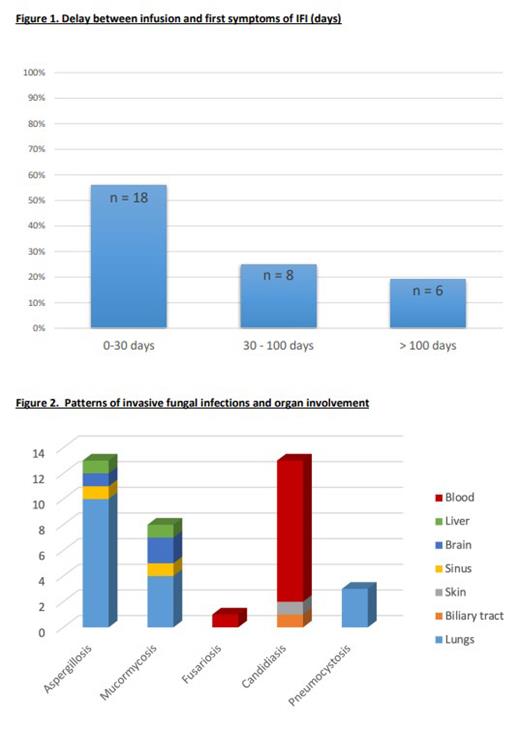
Among the 1144 patients included in the registry from 2018 to 2022, we identified 57 suspected IFI cases. After exclusion of 25 patients not adjudicated for IFI, 32 patients (3%) were identified as having presented a proven (n = 14), probable (n = 12), or possible (n = 6) IFI. DLBCL was the primary diagnosis for 30 patients and axi-cel was the most frequent CAR T product (23/32, 72%). Seven patients (22%) had received a previous hematopoietic stem cell transplantation. All patients experienced cytokine release syndrome (including 8 grade ≥ 3) and 21 (66%) experienced immune effector cell-associated syndrome (including 13 grade ≥ 3). Overall, 27 (84%), 21 (66%), and 5 (16%) received tocilizumab (median 960mg, range 120-2880mg), corticosteroids, and anakinra, respectively. Among known risk factors for IFI, grade 4 neutropenia and late neutropenia (< 1g/L) at day 28 after infusion were identified in 25 (78%) and 14 (44%) patients respectively. Overall, 8 (25%) patients received antifungal prophylaxis, posaconazole was used for 4 of them. Over half of the IFI cases occurred between 0 and 30 days after CAR T-cell infusion (18/32, 56%), with a median onset time of 29.5 days (IQR 16.5 - 79.5). The most frequently observed infections were invasive Aspergillosis (n = 13), with pulmonary involvement in 10/13 (77%) of patients, leading to a fatal issue in 3/13 patients (23%). Other mold infections included Mucormycosis (n = 5), with pulmonary involvement in 4/5 patients and a fatal outcome in 1/5, as well as Fusariosis (n = 1). Yeast infections (n = 12) were mostly identified as Candidemia (92%) with a mortality rate of 3/12 (27%). The median overall survival of patients with IFI was 186.5 days (IQR 79-506), 7 deaths being related to IFI and 3 to lymphoma relapse. Median progression-free survival was 94 days (IQR 31 - 252).
Conclusions:
This study represents one of the largest investigations about IFI in B-cell lymphoma patients treated with CAR T-cells. Even though IFI represent a rare complication after CAR T-cell therapy, the related-mortality is high. Selective antifungal prophylaxis may benefit high-risk patients, while rapid preemptive diagnostic workup, particularly for lung involvement, is mandatory. Analysis to define potential risk factors for IFI are ongoing.
Disclosures
Castilla-Llorente:Nektar Therapeutics: Consultancy; Gilead/Kite: Consultancy, Other: Travel support. Dulery:Ligue Nationale contre le Cancer: Research Funding; Arthur Sachs Scholarships: Research Funding; Servier Foundation: Research Funding; Monahan Foundation: Research Funding; Philippe Foundation: Research Funding; DPC AP-HP: Research Funding; Novartis: Honoraria; Takeda: Honoraria; Kite Pharma / Gilead: Other: Registration fees for scientific meetings and travel accommodations . Di Blasi:Janssen: Consultancy; Novartis: Consultancy, Honoraria; Kite Gilead: Consultancy, Honoraria; abbvie: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; BMS: Consultancy. Herbaux:Physician and professor of Hematology at academic center (CHU Montpellier France): Current Employment; AbbVie, Takeda: Research Funding; AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Honoraria; AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Consultancy. Morschhauser:AbbVie: Consultancy, Other: Advisory Board; BMS: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; Novartis: Consultancy, Other: Advisory Board; Incyte: Other: Advisory Board; Epizyme: Other: Advisory Board; Genmab: Consultancy, Other: Advisory Board; Janssen: Honoraria; Gilead: Consultancy, Other: Advisory Board; Roche: Consultancy, Honoraria, Other: Advisory Board. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Bachy:Novartis: Honoraria, Other: Personal Fees; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Takeda: Honoraria; Incyte: Honoraria; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Kite, a Gilead Company: Honoraria, Other: Personal Fees; Roche: Consultancy, Honoraria. Tudesq:Gilead/Kite: Honoraria; BMS/Celgene: Consultancy. Melica:Pfizer: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Janssen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal